Multineural intelligent system for clinical trials
Optimization, automation and integration on a single platform
Multineural intelligent system for clinical trials
Optimization, automation and integration on a single platform

The Current Problem
- Clinical trial management requires multiple disconnected tools, leading to inefficiency and errors.
- Designing research protocols and extracting variables for CDEs remain manual and error-prone processes.
- Patient data collection through diaries and monitoring systems is often fragmented.
- Compliance with international regulations (GCP, FDA, EMA) requires greater control and traceability.
- Statistical reporting and analysis is a complex and time-consuming process.

The Current Problem
- Clinical trial management requires multiple disconnected tools, leading to inefficiency and errors.
- Designing research protocols and extracting variables for CDEs remain manual and error-prone processes.
- Patient data collection through diaries and monitoring systems is often fragmented.
- Compliance with international regulations (GCP, FDA, EMA) requires greater control and traceability.
- Statistical reporting and analysis is a complex and time-consuming process.
Our Solution...



DROX health science
0
Mission and Vision:
- DROX 360 seeks to revolutionize clinical research by providing an intelligent, secure and fully integrated plataform.
Main features
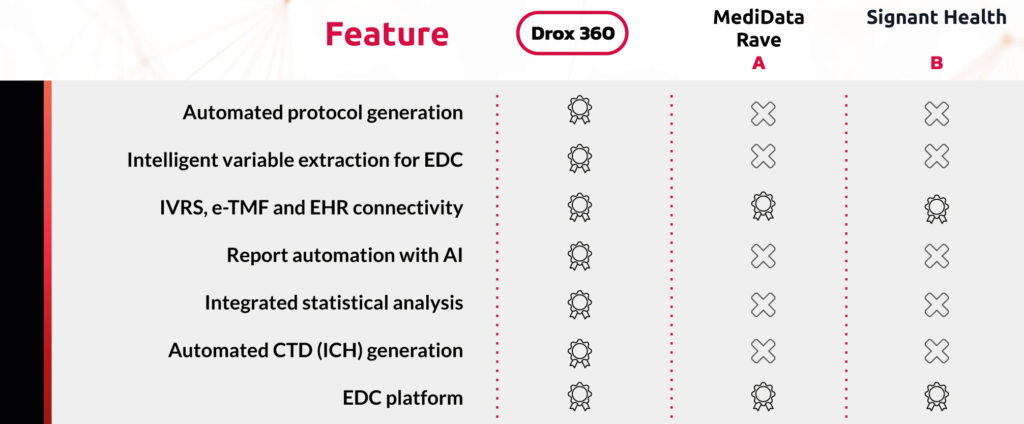
Intelligent Protocol Generation
- AI analyzes data and regulatory guidelines to create optimized protocols.
- Reduced time in the design phase and improved protocol accuracy.
Variables Extraction for EDC
- DROX 360 automates the identification and structuring of variables, accelerating the creation of Electronic Data Capture (EDC).
- Improved quiality and consistency of clinical data.
Study Drug Inventory (IMP)
- Stric control of drug accounting.
- Traceability from the exit of the warehouse.
- Deposit at the research center.
- Assignment to the patient by means of and its return.




Security and Compliance
- Complies with international standards (FDA 21 CCFR Part 11, ICH - GCP).
- Protection of sensitive data with advanced encryption.
Artificial Intelligence Reporting
- DROX 360 processes data collected from visits, diaries anda IVRS to automatically generate the necessary reports.
- AI integrates statistical analysis directly into the platform.
- Automated CTD (Common Technical Document) generation, compliant with ICH.
Regulatory Compliance

Key Benefits of

- Total automation of clinical management, reducing costs and time.
- Reduction of errors in protocol design and data capture.
- Optimized compliance with automated auditing.
- Increased connectivity and integration with IVRS, e-TMF, EHR and mobile applications.
- Cloud accessibility ensuring collaboration from anywhere.
- Automatic generation of reports with AI, including statistical analysis and CTD.

Key Benefits of

- Total automation of clinical management, reducing costs and time.
- Reduction of errors in protocol design and data capture.
- Optimized compliance with automated auditing.
- Increased connectivity and integration with IVRS, e-TMF, EHR and mobile applications.
- Cloud accessibility ensuring collaboration from anywhere.
- Automatic generation of reports with AI, including statistical analysis and CTD.

Leading our partners toward innovation


vs Competition